
HISTORICAL BACKGROUND AND INTRODUCTION
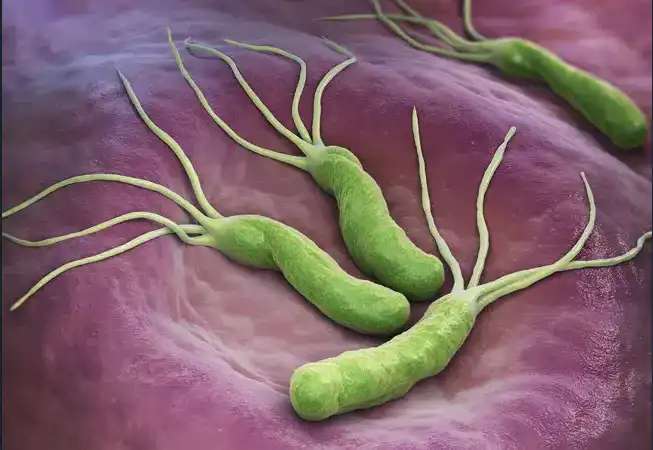
Figure 1: The spiral-shaped Helicobacter pylori bacterium shown here colonizing the stomach surface. Notice the whip-like appendage on the surface – these are called flagella, and is one of the many properties that aid in the survival of the bacterium in the stomach’s acidic environment (image courtesy of the Cleveland Clinic Consult QD website).
Helicobacter pylori, or H. pylori for short, is a common bacterial infection that mainly affects the stomach and the duodenum, which is the first part of the small intestine. The infection is linked to a range of digestive issues. The Helicobacter pylori bacteria was discovered back in 1982 by two Australian doctors, Professor Barry Marshall and Dr. Robin Warren, who took biopsies from patients with peptic ulcers. Their groundbreaking work earned them a Nobel Prize in Physiology or Medicine.

Figure 2: Dr Robin Warren (left) and Dr Barry Marshall (right) won the Nobel prize in 2005 for the discovery of Helicobacter pylori bacterium that changed the landscape of how we view and manage peptic ulcer disease (image courtesy of the Morbidology website).
During that time, the common belief was that stomach and duodenal ulcers were mainly caused by stress and excessive acid production, with infections being overlooked as a potential factor. This viewpoint was based on the idea that no living organism could survive the harsh, highly acidic environment of the stomach. Faced with ongoing failures to culture the bacteria and skepticism from the medical field, Marshall took the bold step of ingesting a broth containing cultured H. pylori bacteria himself. To ensure he had no underlying conditions, he underwent an upper gastrointestinal endoscopy, which came back normal. While he expected symptoms to develop over a longer period, he was caught off guard by nausea, bad breath, and vomiting within just a week. A follow-up endoscopy showed significant stomach inflammation, and biopsy culture tests confirmed that H. pylori was the main culprit behind this inflammation.
This groundbreaking discovery changed the understanding of peptic ulcers, identifying a major new cause of both peptic ulcer disease and gastritis, with H. pylori accounting for up to 90% of small bowel ulcers and 80% of stomach ulcers. Recognizing an infectious origin paved the way for more effective treatment options, including the use of antibiotics, and sparked a fundamental shift in how these conditions were understood and managed. Looking back, we can only imagine how much this seminal discovery positively affected our lives. But work did not stop there as the potential link between H. pylori and stomach cancer led to further research, culminating in 1994 when the World Health Organization (WHO) classified H. pylori as a Group I carcinogen for the stomach. By 2021, H. pylori was once again in the spotlight in the National Toxicology Program’s 15th Report on Carcinogens, which indicated that chronic infection with H. pylori is a known or reasonably anticipated carcinogen in humans.
PREVALENCE
In Malaysia, the rates of H. pylori infections are modest, with local studies indicating that about 13.5% of the population is affected. However, some regions grappling with challenges like overcrowding, poor sanitation, and hygiene issues, may experience higher infection rates. Interestingly, several research efforts in Malaysia have found that individuals of Indian and Chinese descent generally show higher infection rates compared to Malays, though the reasons behind this remain unclear. Other factors, such as age, gender, diet, environment, and cultural practices associated with different ethnic groups, can also play a role in infection and transmission rates. For instance, one study from Kelantan highlighted that a diet incorporating 'budu' (a traditional anchovy sauce) and 'pegaga' (also known as Centella asiatica) is associated with a reduced risk of contracting H. pylori. Whether we should start incorporating more of these food in our diet remains questionable as there are more to what meets the eye.
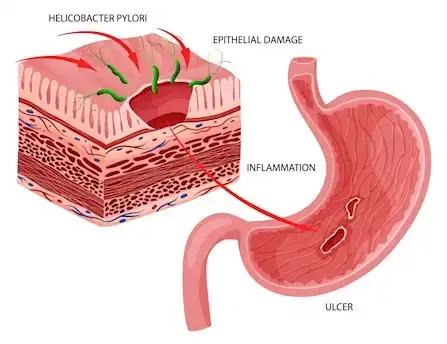
Figure 3: Cartoon of the Helicobacter pylori bacterium inducing long-standing inflammation and ulcers on the surface of the stomach (image courtesy of the Australian Clinical labs website).
BACTERIAL PROPERTIES
Helicobacter pylori is pretty unique because it can survive in the super acidic environment of the stomach, which most other bacteria can’t handle. It produces an enzyme called urease that breaks down urea—the body’s waste product—into ammonia. The ammonia compound is alkaline and helps neutralize the stomach acid, which is key for the bacteria's survival. However, it can also cause damage to the stomach lining. When the protective mucus layer gets eroded, the stomach tissues underneath can be exposed to the harmful substances that H. pylori produces.
On top of its metabolic properties, H. pylori has a few fascinating physical features. It has 5 to 7 whip-like flagella that let it navigate around easily in the stomach's upper layers. Plus, it creates special proteins that help it stick to the stomach walls, which allows it to settle in and trigger a significant inflammatory response. Left undiagnosed and untreated, the ongoing damage can lead to stomach and small bowel ulcers and might even cause gastrointestinal bleeding.
HELICOBACTER PYLORI AS A CARCINOGEN
Through various pathological processes, H. pylori can play a role in the development of stomach cancer by triggering ongoing inflammation, releasing toxins, causing DNA damage and mutations, altering the gut microbiome, and interacting with environmental risk factors like smoking, alcohol use, and particular dietary habits. The Centers for Disease Control and Prevention (CDC) recognizes H. pylori as a carcinogen and a significant risk factor for cancer, estimating that infected individuals may face up to a 20-fold increased risk of developing stomach cancer compared to those who are uninfected.
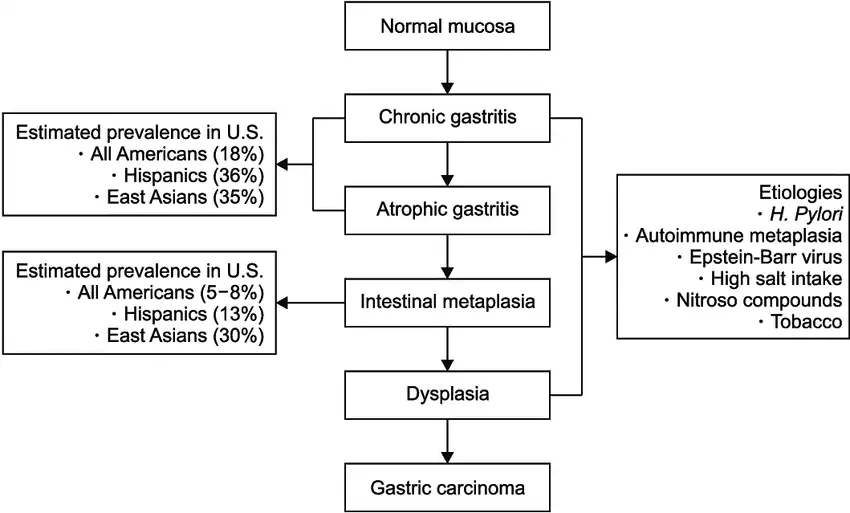
Figure 4: Step-by-step progression of how the Helicobacter pylori infection triggers ongoing changes on the stomach lining, from mild inflammation all the way through to stomach cancer (image courtesy of Huang et. al Gut and liver 2019).
SIGNS AND SYMPTOMS
Patients infected with H. pylori can exhibit a variety of symptoms, which may differ in intensity. While some individuals may remain asymptomatic, others might experience burning or cramp-like abdominal pain, nausea, vomiting, foul breath, bloating, frequent burping, diarrhea, loss of appetite, weight loss, and unexplained fatigue. In more severe cases, patients may show concerning signs such as passing bloody stools, black tarry stools, or vomiting blood. It's crucial to understand that not every symptom needs to be present at the same time to identify an H. pylori infection. Additionally, these symptoms aren’t solely indicative of an H. pylori infection, as other potential diagnoses should be considered. On the flip side, some patients may report atypical symptoms, such as a sensation of a painless lump in the throat (known as globus), prolonged coughing, chest discomfort, sore throat, throat irritation, headaches, joint pain, symptoms related to the thyroid, and various skin rashes.
THE TRANSMISSION OF H. PYLORI INFECTION
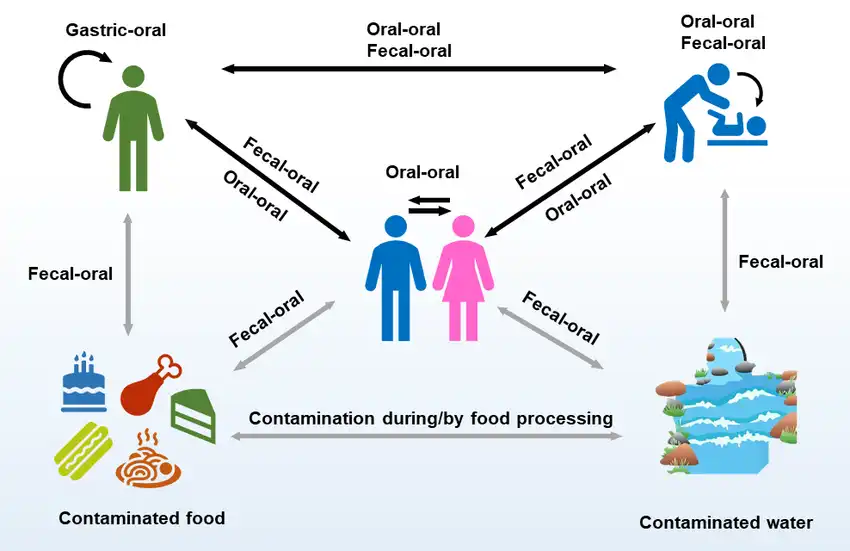
Figure 5: Potential transmission route of Helicobacter pylori infection (image courtesy of Almashhadany et. al Italian Journal of Food Safety 2024).
Helicobacter pylori spreads from one person to another through direct contact with saliva, vomit, or fecal matter. This transmission can happen when people share utensils, kiss, or come into contact with vomit or stool from someone who is infected. Those infected can pass H. pylori in their feces, and if this contaminates food or water, others can ingest it and become infected. Living in overcrowded settings and having poor hygiene practices, especially during childhood, can increase the chances of H. pylori infection and potential reinfection.
It’s important not only to treat the infected person but also to check close contacts to significantly lower the risk of reinfection. Generally, the chance of H. pylori reinfection after successful treatment is relatively low, particularly in developed countries. Annual reinfection rates in adults and children over five typically range from about 0.5% to 2.0%. In contrast, in developing nations, these rates can exceed 10%.
DIAGNOSIS
Blood / Serology test
Figure 6: Bloods for serology to detect the Helicobacter pylori may not always be accurate in identifying the present status of an infection (image courtesy of the National Heart, Lung, and Blood Institute website).
Blood tests can detect the presence of antibodies against H. pylori. However, one drawback of these tests, compared to other diagnostic methods, is their inability to distinguish between active infections and those that have been cleared, as antibodies can linger in the bloodstream long after an infection has resolved. Other diagnostic approaches, like breath tests, stool antigen tests, and upper endoscopy, are generally much more effective at pinpointing active infections. In some rare cases, blood tests may produce false-positive results. This can happen if the immune system generates antibodies against bacteria that look similar or if there’s a mistake during the testing process. As a result, H. pylori IgG (blood or serology) tests are not considered the most reliable method for diagnosing acute H. pylori infections.
Urea Breath Test

Figure 7: Performing the urea breath test is quick, economical, accurate and safe (image courtesy of the Suburban Gastroenterology. Ltd website).
The urea breath test utilizes the urease enzyme, produced by H. pylori, to break down urea into carbon dioxide and ammonia. During the test, an individual consumes urea tablet labeled with a radioactive or stable carbon isotope, like 13C. By measuring the amount of labeled carbon dioxide exhaled, healthcare providers can determine the presence and activity of H. pylori.
To ensure accurate results from a urea breath test, it’s essential to follow specific preparation guidelines. First, individuals should fast for at least 4 to 6 hours beforehand. It's also crucial to refrain from smoking for at least 30 minutes prior to the test and to avoid taking certain medications.
Antibiotics: It’s necessary to stop all antibiotics for a minimum of four weeks before the test. This includes medications such as bismuth subsalicylate, clarithromycin, metronidazole, levofloxacin, amoxicillin, rifabutin, and tetracycline (doxycycline). The presence of antibiotics can hinder the urease enzyme's activity, potentially leading to a false-negative result.
Proton Pump Inhibitors (PPIs): Medications known as PPIs—such as omeprazole, pantoprazole, esomeprazole, lansoprazole, rabeprazole, and dexlansoprazole—should be discontinued at least 1 to 2 weeks ahead of the test.
Potassium-Competitive Acid Blockers (P-CABs): If you're taking P-CABs, like vonoprazan, these should be stopped at least 5 days before the test. Similar to PPIs, they reduce stomach acid production, which can interfere with H. pylori's urea metabolism and may affect the accuracy of the test results.
During the procedure, breath samples are collected both before and after consuming the labeled urea. A notable increase in the concentration of labeled carbon dioxide in the exhaled breath indicates a positive result, confirming the presence of H. pylori. Typically, results from the urea breath test are available within 24 to 48 hours, although certain centers equipped with automated machines may provide results within just a few hours.
Fecal / stool antigen test
Figure 8: The stool antigen test is an accurate method to detect Helicobacter pylori infection, similar to that of a stool occult blood test kit (image courtesy of the Prima website).
The fecal antigen test is designed to detect H. pylori antigens in stool samples. Similar to the urea breath test, it’s crucial to follow the provided instructions closely, which may include specific guidelines for collecting the stool sample, such as using a special collection device or avoiding certain medications prior to the test (as is recommended for the urea breath test). In the lab, a technician usually applies a method known as ELISA (Enzyme-Linked Immunosorbent Assay), which uses antibodies specifically linked to H. pylori to determine whether the bacterial antigens are present in the stool. A color change or other visual cue signals the presence of these antigens. However, due to concerns regarding hygiene and sanitation, the stool antigen test is not widely accessible in Malaysia.
Endoscopy with biopsies
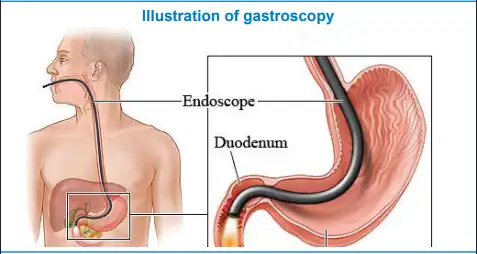
Figure 9: Illustration of the esophagogastroduodenoscopy procedure (image courtesy of the NHS Foundation trust website).
An endoscopy, specifically an esophagogastroduodenoscopy (EGD), is performed to diagnose H. pylori infection by collecting tissue samples from the stomach. These samples are then analyzed using a test strip, such as the Pronto dry and rapid urease test, for quick results on-site. Before the procedure, routine patient preparation takes place, which includes administering a throat spray (lignocaine) to numb the back of the throat. A bite block is inserted between the teeth, and a nasal cannula is placed to provide supplementary oxygen.
While sedation is generally offered in our practice, it's important to note that the procedure can be done without it if preferred. Patients have the option to choose their method of sedation. It's widely agreed that sedation can improve the overall experience by alleviating anxiety and fear, enhancing satisfaction, comfort and tolerance, and helping the procedure go more smoothly. The EGD typically lasts around 7 to 9 minutes, with biopsies collected at the end to confirm the H. pylori infection.
Afterward, patients will spend about 30 to 45 minutes recovering in the endoscopy suite, with longer monitoring if necessary. Once they're ready, they will be taken back to their day care wards for complete recovery. Most centers provide a light snack, such as a sandwich, yoghurt, juice or porridge, and your doctor will discuss the results with you before you head home.
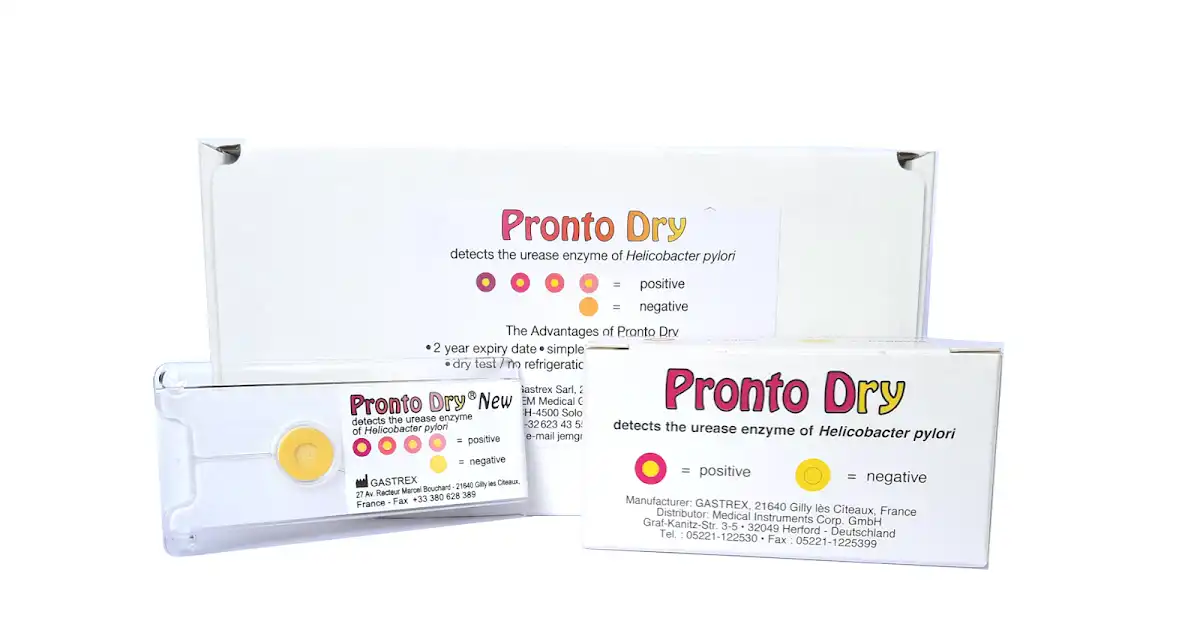

Figure 10: Test kits where tissue samples from the stomach are placed; top panel – the Pronto dry (image courtesy of the Kokab Enterprise website) and bottom image – the CLOtest or rapid urease test (wet) by Kimberly-Clark – a positive test is indicated red on the specimen pot (image courtesy of the Malaysian Medical Gazette website).
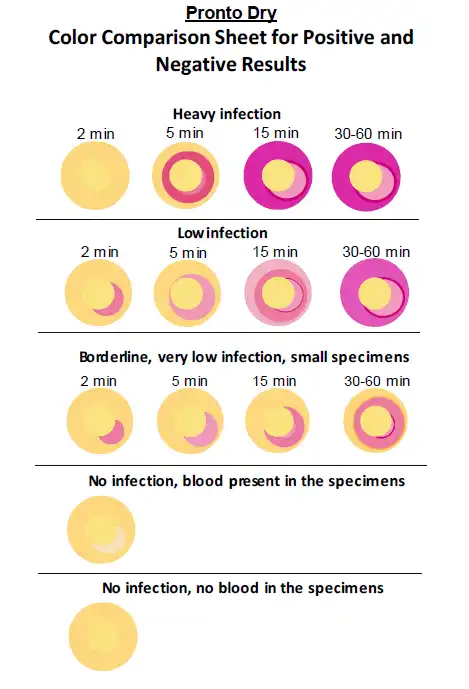
Figure 11: The interpretation guide of the test kit revealing positive or negative results (image courtesy of the AnaChem Diagnostics website).
TREATMENT AND PREVENTION
When an H. pylori infection is confirmed, treatment usually involves a combination of an acid-suppressing agent—most often a proton pump inhibitor (PPI), or more recently, a stronger option known as PCABs—and two different classes of antibiotics over a course of 1 to 2 weeks. It's essential for patients to stick to their prescribed medication regimen and take the medications on time to ensure the best results. Common side effects of antibiotics can include a bitter tongue, headaches, dizziness, drowsiness, nausea, vomiting, reduced appetite, bloating, abdominal discomfort, and diarrhea. Patients should inform their healthcare providers about any allergies that might require a switch to a different class of antibiotics. It's also worth noting that treating H. pylori infection is becoming increasingly complicated due to antibiotic resistance, and the development of new treatments has not kept pace with the growing resistance seen in these bacteria. Thus, adhering strictly to prescribed medications is of paramount importance.
Recently, the introduction of a fungal-based probiotic, Saccharomyces boulardii, into standard treatment protocols has shown promise. It has been linked to improved eradication rates and reduced side effects, such as nausea, vomiting, and diarrhea, associated with antibiotics, as well as better overall patient compliance. This newer approach is worth considering in light of the ongoing challenges posed by antibiotic resistance.
As with all other illnesses, preventive care is better than having to contend with a cure that is mired with potential obstacles. While there is currently no vaccine for H. pylori infection, adopting good hygiene practices—such as washing hands regularly with soap, using hand sanitizers, consuming thoroughly cooked foods, and drinking clean water—can significantly help prevent infection.
IMAGE TRIVIA OF ENDOSCOPIC APPEARANCE IN HELICOBACTER PYLORI INFECTION
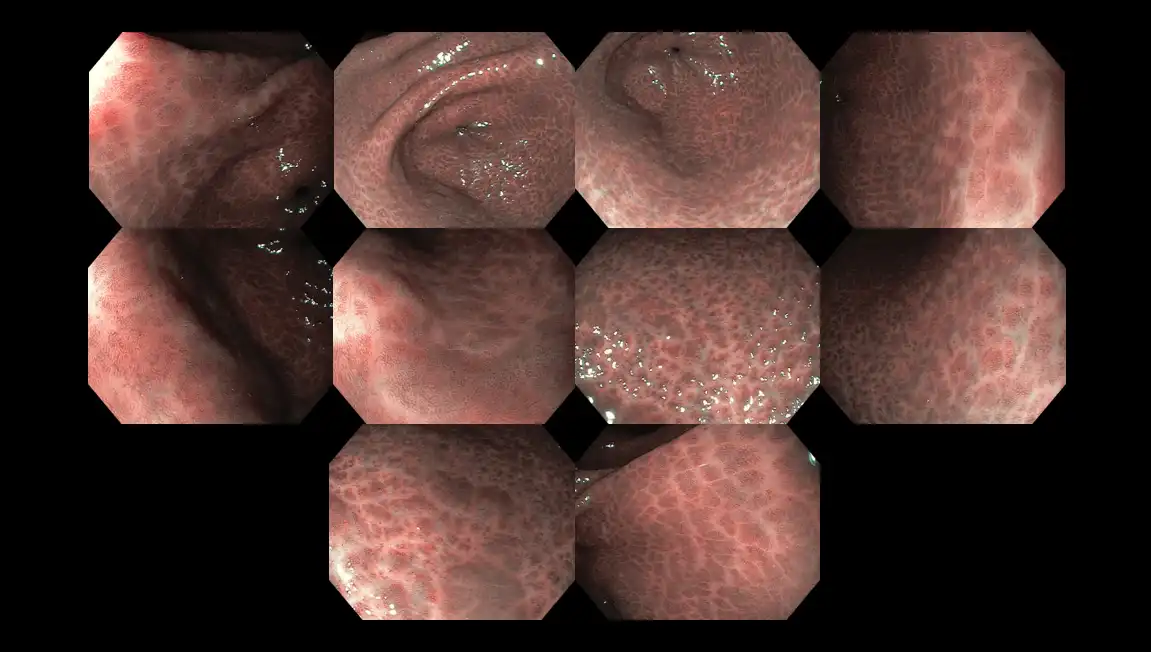
Figure 12: Surface appearance of the stomach wall infected with Helicobacter pylori bacteria exhibiting the classical ‘snake-skin’ appearance. The whitish areas are scarred tissue whereas the blackish marks developed due to long-standing inflammation.
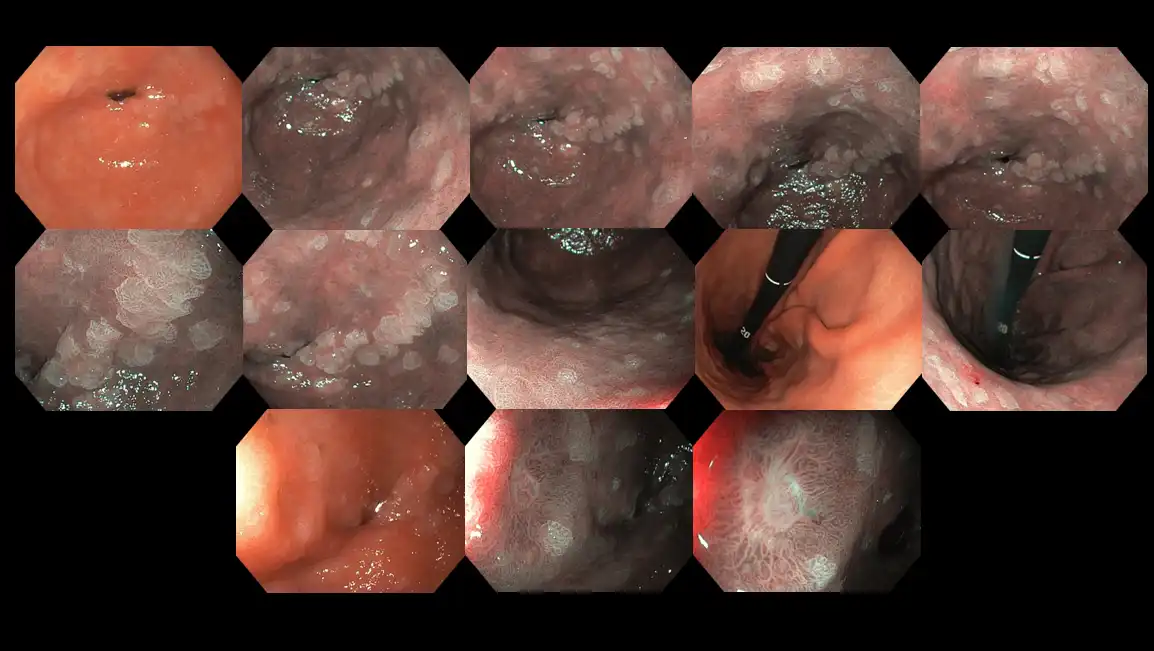
Figure 13: High-risk endoscopic appearance of a stomach infected with Helicobacter pylori bacteria. The silvery scale-like marks are called ‘intestinal metaplasia’ and they harbor an elevated risk of transforming into cancer cells (see Figure 4). The last row depicts how a stomach ulcer looks like.
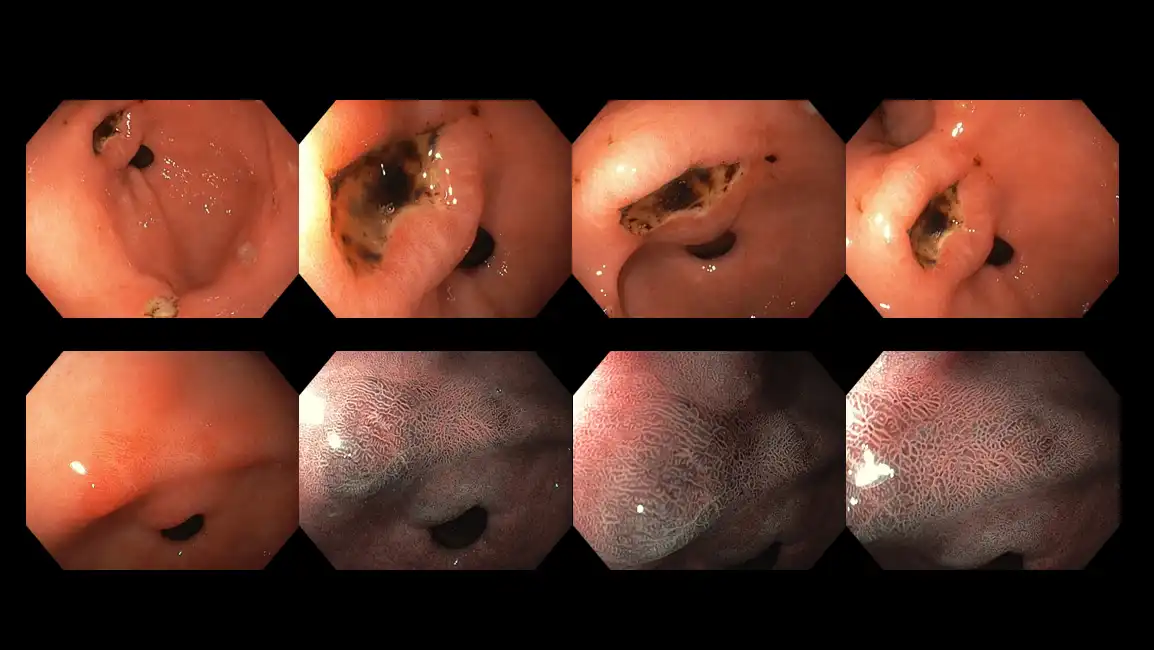
Figure 14: Image illustrating a stomach ulcer – the blackish marks are dried-up blood clots and points to an area where gastrointestinal bleeding has occurred recently.
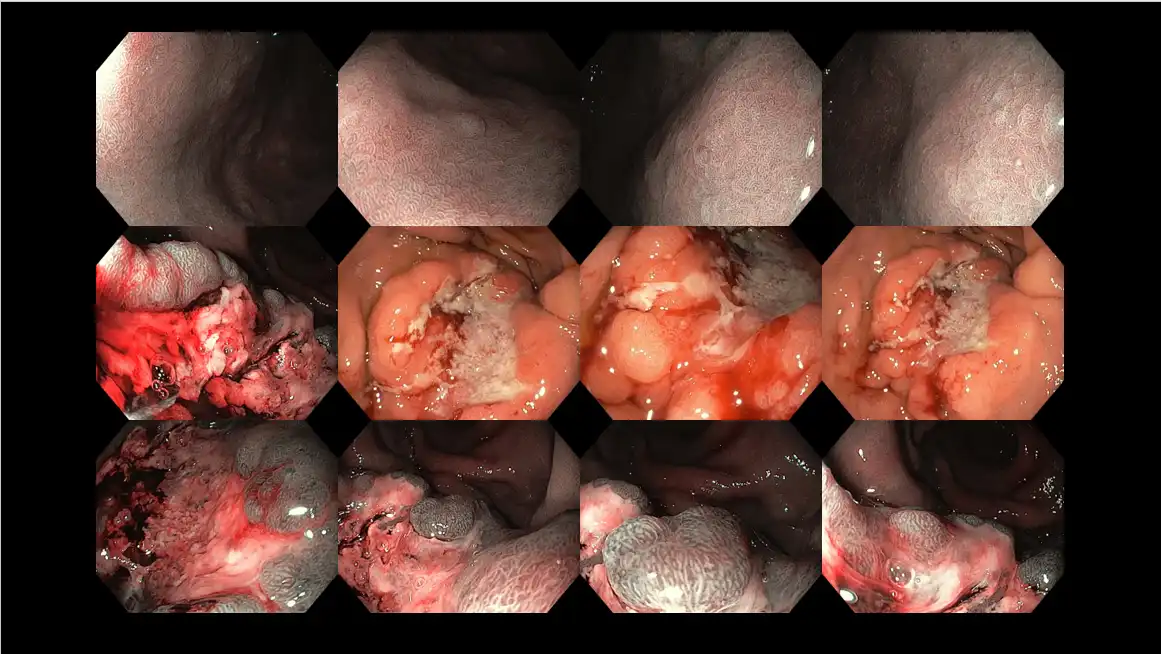
Figure 15: Stomach cancer attributed to Helicobacter pylori infection in a middle-aged patient presenting with several months of nausea, vomiting, abdominal discomfort, appetite and weight loss. The top row shows features of long-standing infection, whereas the middle and bottom row demonstrates the area of stomach cancer with a huge ulcer crater in the middle.
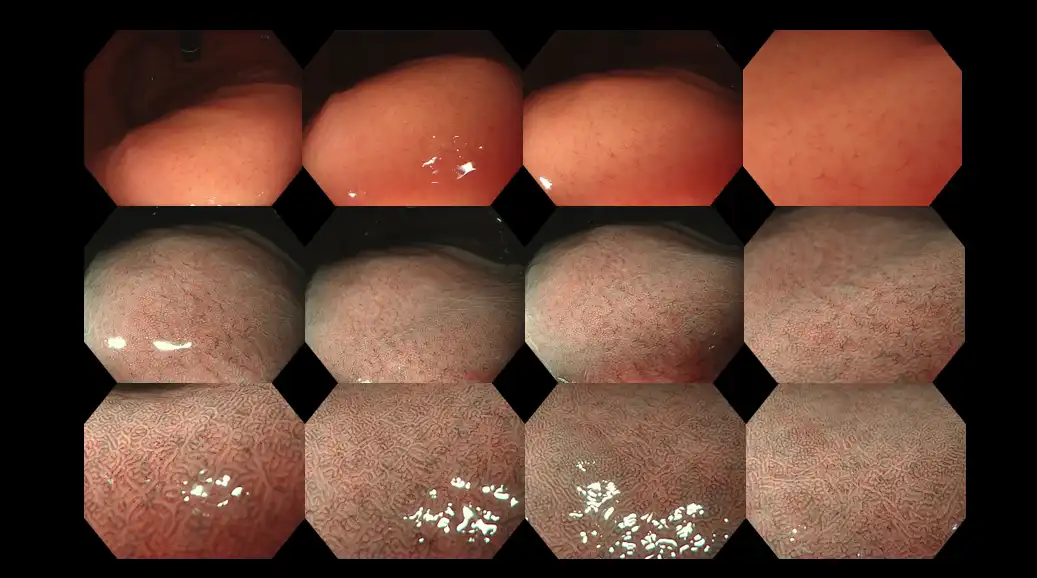
Figure 16: Ultra high-definition and high-magnification gastroscopy in a patient without the Helicobacter pylori infection. As a baseline, such findings represent a normal study. Zoom gastroscopes are widely used in Japan, as a means to diagnose H. pylori infection and delineate tiny early-stage cancer, sometimes as small as 1-2 mm.


